Conductivity
Research
- Long-term reproducibility: How well does this probe stay accurate? (Dr. Conrad Gregoire)
- Comparison to professional probes: How well does our testkit compare to professional equipment? (Carleton University, Conservation Authorities)
What is conductivity?
Conductivity is how we measure the ionic content (such as chloride, nitrate, sulfate, sodium, magnesium, calcium, or iron) in a body of water by measuring the water’s ability to conduct electricity.
Why is conductivity important?
Every body of water has its own unique conductivity level, based on its bedrock. It is important to establish a baseline as some bodies of water have naturally high levels due to their geology and geography.
What does a conductivity measurement mean for water?
We can use conductivity as an early warning system for potential problems that warrant further testing. If we see a variance (either lower or higher) from our baseline readings, we know something may have gone wrong. While lab testing is the usually the only way to determine what is causing the reading, higher readings could indicate a pollution event has taken place.
What about ocean water? Conductivity reading for ocean water is about 55,000 µS. While some people test conductivity in sea water, many meters won’t read that high. Many protocols choose to measure salinity instead. Salinity is measured in parts per thousand, with the average ocean reading measuring 35 ppt. Just like with all tests, we can convert readings, although conductivity measures everything that conducts electricity, while salinity only measures salt.
Some common values
| Distilled water | 0.5 – 3 µS |
| Melted snow | 2 – 42 µS |
| Has effects on fish reproduction | over 500 µS |
| Tap water | 50 – 800 µS |
| Potable water | 30 – 1500 µS |
| Freshwater streams | 100 – 1,000 µS |
| Industrial wastewater | 10,000 µS |
| Sea water | 55,000 µS |
E.g. Most readings for the Ottawa river are below 100 µS, but near a storm sewer could have conductivity values over 2,000 µS (or 2.0mS).
Ask an expert
Conrad Gregoire, Chemist, introduces Conductivity
Water Rangers Testing Protocol
Note: old models have a cap you need to remove. If you purchased your meter after 2021, you will not have a cap.
- Turn on the meter by pressing the top button.
- Dip the meter into the water. Do not dunk the whole device in as the battery is near the top. Hold in the water for 2 minutes, swishing it around lightly. Continue until both values remain steady for 30 seconds.
- Tip: If you get a reading of 10 or less, you have not removed the cap or you have taken the sensor out of the water before reading) Read the measurements. We record in µS/cm (microsiemens per centimetre), so check the units. If you get a reading like 1.3, it is converting it to mS (millisiemens) and you must multiply 1.3 by 1000 and record 1,300 in your form.
- Make sure you turn off the device after use to preserve battery life (top button).
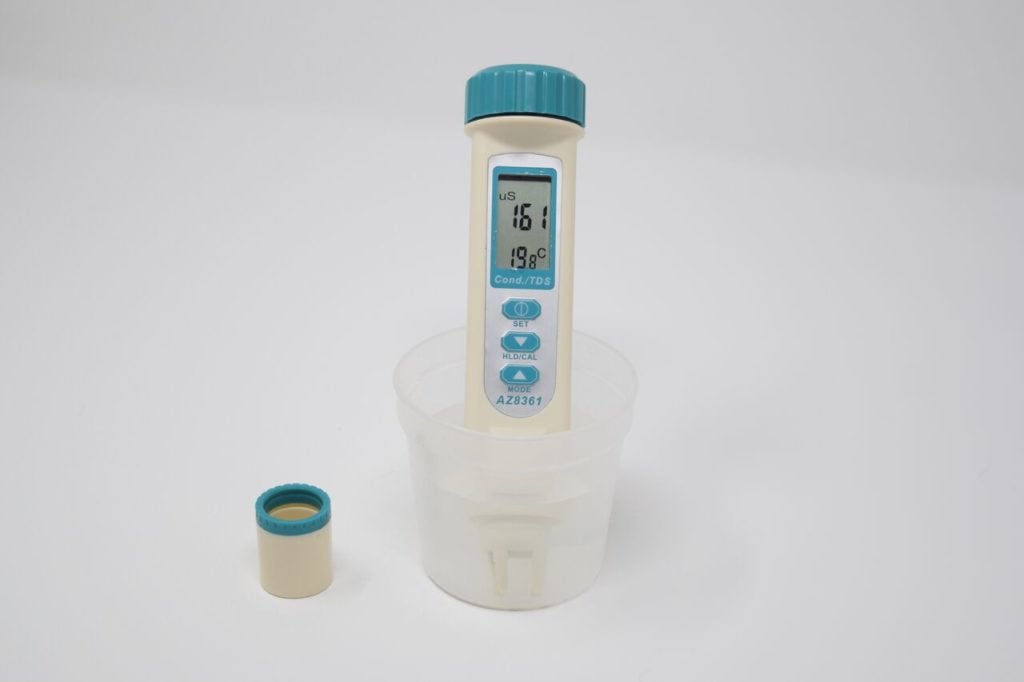
How to test for conductivity
Taking care of your conductivity meter
Since the probe is conducting an electric current, it is so important to keep the electrode clean. Rinse electrodes in distilled water often, especially if you are taking samples where readings are high or if the area is polluted.
Calibration
Learn how to calibrate your meter here. Please note, one pouch is available to those who have purchased a testkit. For additional pouches, visit our store.
Ask an expert
Conrad Gregoire, Chemist, how to test and use a reference to make sure you’re accurate.
Get started with testing conductivity here:
-
Compact Freshwater Testkit$140.00
-
Conductivity meter$60.00
-
Freshwater conductivity calibration solution$4.00
Contributing to the community!
Water Rangers is citizen-scientist led. So, if you have any questions, ideas, or notice any errors, please tell us!
Parameter FAQs
Check out out parameters page for an in depth description and protocol to our testing parameters!
Water Rangers conductivity meters measure the conductivity of freshwater in µS, or microsiemens.
Water will measure different results of conductivity depending on it’s condition, shown below;
| Distilled water | 0.5 – 3 µS |
| Melted snow | 2 – 42 µS |
| Has effects on fish reproduction | over 500 µS |
| Tap water | 50 – 800 µS |
| Potable water | 30 – 1500 µS |
| Freshwater streams | 100 – 1,000 µS |
| Industrial wastewater | 10,000 µS |
| Sea water | 55,000 µS |
For more an introduction, our protocol, and additional information regarding conductivity, we encourage you to visit our conductivity parameter page!
Water Rangers measures dissolved oxygen, or DO2, in milligrams/litre.
Different measurements of DO2 will mean different circumstances for life in a water body, shown below;
- 0-2 mg/L: not enough oxygen to support life
- 2-4 mg/L: only a few fish and aquatic insects can survive
- 4-7 mg/L: good for many aquatic animals, low for cold water fish
- 7-11 mg/L: very good for most stream fish
For more an introduction, our protocol, and additional information regarding dissolved oxygen, we encourage you to visit our dissolved oxygen parameter page!
For optimal results, E.coli tests require an incubator. We don’t currently sell supplies that test for E.coli, but we may one day. Learn more about E.coli here.
If you’re looking for an E.coli test, we recommend TapScore.
Just like with phosphates, this is another parameter where the tests we’ve tried aren’t sensitive enough unless you’re in a pollution event. Probes we’ve seen don’t seem very sensitive, and experts we’ve worked with aren’t sure how accurate they are.
We encourage our testers to browse our affiliate Tap Score’s testing services, which include products for phosphates!
We are currently conducting research involving tests for high level phosphates, but have yet to find tests that we’re happy with. Field tests that are currently available on the market can you let you know if phosphates are present, but can’t tell you to what extent they are present. In other words, they’re not quite sensitive enough to be useful! Also, many of the field tests require you to handle reagents that can be toxic, and that makes disposal difficult, meaning they wouldn’t be safe for kids to use. We are watching this space closely, and working with partners to see if this can change.
While our kits are designed to test lakes, rivers, and oceans, parameters like lead, copper, calcium, and unwanted chemicals are critical for determining the overall safety of city water. We promote groups like Tap Score who have created easy-to-use monitoring kits for your home.
Tap Score has designed kits to test city water and well water, depending on the source of your water. They provide you everything you need to collect a sample and send it to them. Upon analyzing it, a water health report will be returned to you in only a matter of weeks!
If you’re looking to test the quality of freshwater, visit our store for the appropriate testing supplies!
To learn more, check out our parameters page on pH!


