Chloride
What is it? Chloride is a negatively charged ion that is formed when chlorine gains an additional electron. It is often found in the form of sodium and potassium salts, which are very soluble in water. Road salts are a common example, which spike chloride levels.
Why is it important? Almost all natural bodies of water contain chloride, but the concentrations can vary depending on the mineral content of the surrounding rock or soil. Chloride also enters the water from human sources including: agricultural runoff, road salts, industry wastewater, among other things. Our new winter testkits are particularly concerned with road salt contamination.
What does it mean? Testing for this parameter can be one way of measuring pollution in water. Above normal concentrations can significantly impact plant and aquatic life. Some plankton are very sensitive to large concentrations and without these little guys to eat the algae, we see significant problems like lack of dissolved oxygen. Chloride is also an important indicator of road salts and we monitor this parameter in our winter testkits. Road salt contamination can have a range of impacts on aquatic ecosystems, drinking water, infrastructure, and so much more. Learn about the impacts of road salt contamination here!
Water Rangers testing protocol: Check out our new winter testkits to begin monitoring road salt contamination in your local waterbodies! We use HACH teststrips to test for chloride.
- Collect your water sample using your throw bucket or reacher stick
- Shake out a chloride teststrip and close the bottle
- Dip the strip into your sample up to the 6 line
- The orange strip at the top of the teststrip will turn black when the test is complete. This will take about 3 minutes
- The white line going up the strip will indicate the chloride level of your sample
- Take the reading from the closest line to the white and compare it to the graph on your sample bottle
- Record your results in parts per million (ppm). Note: ppm=mg/L
Chloride guidelines
Freshwater lakes and rivers naturally contain low concentrations of chloride. Freshwater generally has chloride concentrations below 100mg/L. Note: ppm = mg/L.
Salt chronic exposure guideline: 120 milligrams of chloride per litre of water (120 mg/L or 120 ppm).
Salt acute exposure guideline: 640 milligrams of chloride per litre of water (640 mg/L or 640 ppm).
Reading your results
Every batch of chloride strips will have a slightly different comparison chart.
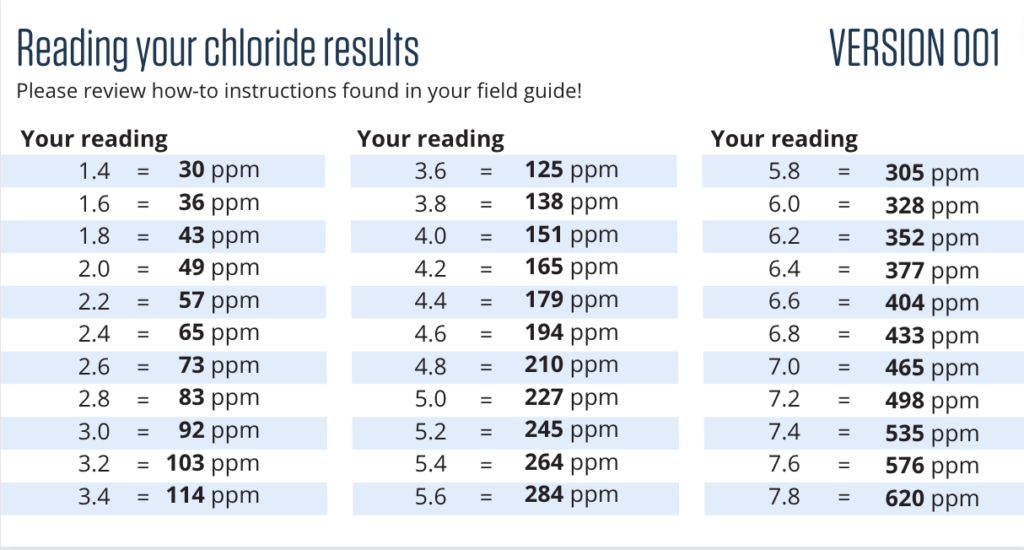
How to:
- For accuracy, pull out the chart that matches the version number on your chloride strip.
- Take the chloride reading.
- Compare the reading number to the chloride level in parts per million (ppm).
- Record your reading in your notebook or on our IOS app.
Chloride & conducivity
Chloride and conductivity are two related parameters that we monitor in our winter testkits. While chloride is a direct measure of road salt contamination, conductivity as an indirect way to measure concentrations. Road salts (sodium chloride) often cause massive spikes in conductivity in the winter and spring. We can use conductivity as a reference for chloride levels.
Get started monitoring road salts here:
-
Chloride test strips – 10 pack$32.50
-
Winter Testkit$165.00
-
Conductivity meter$60.00
Contributing to the community!
Water Rangers is community-led. So, if you have any questions, ideas, or notice any errors, please tell us!
Extra resources
This Illinois group has been monitoring chloride for the last few years. Check out their dataset here!
Here is another blog post about conductivity and salinity!


