pH in saltwater
What is pH in saltwater?
pH stands for “potential for Hydrogen”. It is the measure of the acidity or alkalinity of saltwater.
Why is pH important in saltwater?
pH sets up the conditions for how easy it is for nutrients to be available and how easily things like heavy metals (toxicity for aquatic life) can dissolve in the water. Rivers and lakes generally range between 5 (acidic) and 9 (basic) on the pH scale, whereas ocean water averages closer to 8.2 (slightly basic). Low pH can reduce shellfish’s ability to reproduce and grow thick shells.
What does a pH measurement mean for saltwater?
The most important thing is to first establish a baseline for testing. Based on that number we can determine if something is influencing the water’s health.
pH Fast facts
- Changes in pH could indicate that an area is in trouble
- Plants affect pH through photosynthesis and respiration: pH is highest
in the afternoon. - The ocean’s average for pH is around 8, but it varies, depending on your location.
- Surface pH can be much higher than at depth.
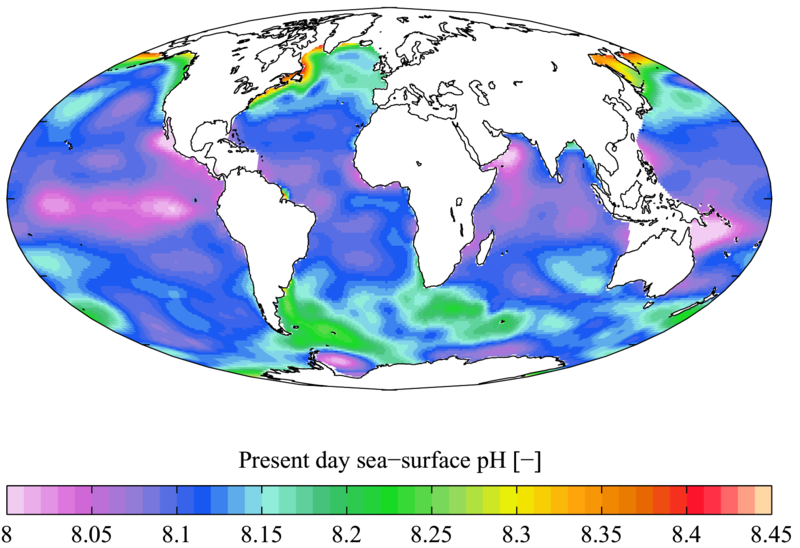
- The ocean’s pH is getting lower due to ocean acidification caused by carbon sequestration. The Atlantic Ocean has seen a big decrease in pH since the 1700s.
- Lower pH can have an effect on animal’s abilities to grow, specifically their shells. Animals most affected by lower pH: shellfish, corals, plankton, sea urchins, and fish.
Water Rangers protocol for pH in saltwater
We use test strips designed for marine aquariums. You cannot use freshwater strips for salt water. We do not sell the test strips on their own, but we recommend this brand on amazon. We have yet to do a complete comparison of all test strips, but preliminary reports show that these ones are great!
Looking to test pH in saltwater? Check out this kit:
-
Ocean Explorer Testkit$360.00
Further reading
- What is Ocean Acidification?: Department of Fisheries and Oceans
- Ocean Acidification: Really thorough explanation with resources from the Smithsonian
Contributing to the community!
Water Rangers is citizen-scientist led. So, if you have any questions, ideas, or notice any errors, please tell us!
