Lake McGlashan water chemistry

Lake McGlashan, located in Val-Des-Monts, Quebec, is a beautiful clear, deep freshwater lake. It is more than thirty meters deep at its deepest point and connected to its neighboring water body, Lake Girard, through a small stream.

Lake McGlashan is surrounded by steep hills, both on the North and South sides, protecting it from the preeminent winds. On the South side, behind the mountain, a large network of streams and wetlands with many beaver ponds constitutes the principal lake’s drainage basin.
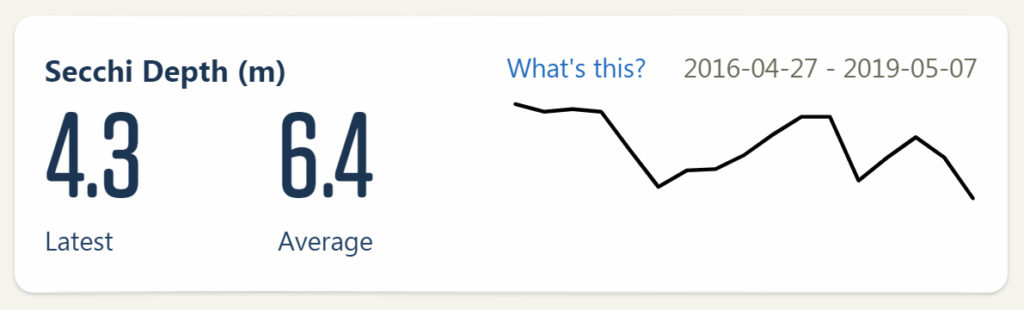
It is classified as a young, oligotrophic lake, meaning that the water is very clear, has sparse plant growth, and it has low nutrients. We take monthly water quality samples of surface water. You can view the data here.
Taking ‘profiles’ in water quality


In 2017 and 2018, our founder, Kat Kavanagh, went out with Stéphanie Milot from the Fédération des Lacs de Val-des-Monts to take a ‘profile’: that’s when we lower a probe into the water and record the values at every meter for temperature, pH, conductivity, and dissolved oxygen. The probe could measure 30 meters of the 35-meter depth in the lake’s deepest point, with some incredibly interesting results! At different depths, the lake has distinct water chemistry, which has led some of our partner limnologists (limnologists = lake scientists) to determine that it is meromictic.
What is a meromictic lake?
Most lakes’ water mix completely during a typical year. However, a meromictic lake does not, and a portion of the waterbody remains distinctly different. One of the most famous meromictic lakes is Pink Lake, located in Gatineau Park, where water at its bottom has remained there since the last ice age (and is very salty!). Meromictic lakes are rare: research shows they’re one in every one thousand lakes. So, finding out that Lake McGlashan is meromictic is very exciting!
We’ll walk through the different parameters we measured. It is important to take profiles during different seasons to understand the changes.
Temperature
Normally, throughout the seasons, the deeper you go, the cooler the temperatures become because there is little to no sunlight to warm the water at great depths. However, at Lake McGlashan, after going down 15 meters from the surface, the water temperature becomes constant at 4 degrees Celsius.
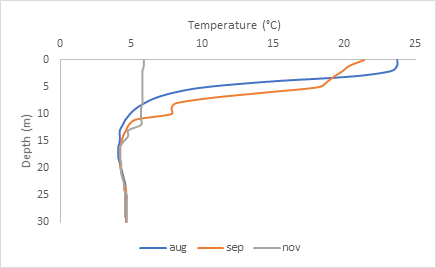
As shown on the graph above, the top 15 meters of water change depending on the season. In August and September (between 20-25 degrees Celsius), the temperatures in the top layer are quite warm, but in November, it is around 6 degrees Celsius. This top layer is called Epilimnion, and the 15-meter mark, which acts as a boundary between the top and bottom layer, is called Thermocline. The bottom layer where the temperature becomes constant across the board is called Hypolimnion. The formation of layers, called stratification, often occurs in deep lakes. Temperature isn’t the only parameter that is different between layers.
Dissolved oxygen
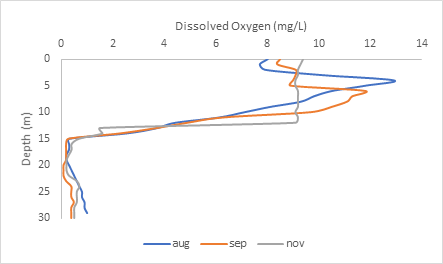
You can see above the thermocline, that dissolved oxygen is very high: between 8-13 mg/L between August to November. But after the thermocline 15-meter boundary, the water becomes anoxic, meaning no oxygen is found at the bottom layer of the lake.
Why is there no oxygen at the bottom of the lake?
Organisms like fungi and bacteria, like to break down organic matter that accumulates at the bottom of the lake. They use up all the dissolved oxygen for microbial decomposition, and there is no atmospheric oxygen or oxygen from photosynthesis there to replace it.
Lake McGlashan does not support large fish populations, but you can find Sunfish, Bass, Pike, and Minnows in the top layer of the water. You will not find fish at the hypolimnion (bottom layer) because it does not have the oxygen fishes need in order to breathe.
For example, trout need oxygenated water at a concentration of at least 4ppm. Trout need places where the dissolved oxygen level is consistently high, or the water temperature is consistently cool, or where there is some happy combination of both. Because of the water chemistry, fish like trout could not prosper in Lake McGlashan.

Can you say meromictic? Understanding conductivity readings
The conductivity also changes drastically between the different layers. In the top Epilimnion layer, there are few ions (approximately 100 µS/cm), but at the bottom layer, the conductivity spikes to about 900 µS/cm! Minerals and ions partly come from the lake’s bottom rocks and soil. This enriches the conductivity at the bottom of the lake. Although spikes in conductivity reading can sometimes be an indicator of a pollution event, in this case, particles like salts, minerals, and metals accumulating at the bottom are natural for this lake. Stéphanie and Kat specifically retested in November (in the snow!) and in May of 2018 to ensure that the barrier at 21 meters was stable. When experts look at these conductivity readings, along with low oxygen readings, they conclude that the lake is meromictic: the bottom layer does not mix with the rest of the lake water like a lake typically does.

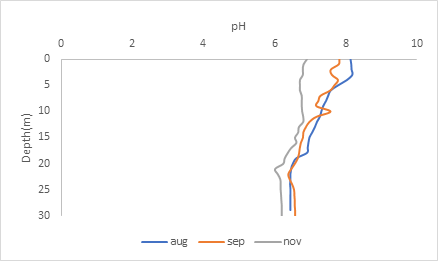
pH
The graph shows that the lake becomes slightly acidic as the season becomes cooler. But in general, the pH does not change drastically because there are particles in the water called buffers that keep the pH relatively within range. You’ll notice that after the thermocline, there is little variation in the pH at the lower depths. This is again due to lake stratification, where there are distinct layers in the water that do not mix.
Lake stratification occurs because the variation in temperature in the different depths of the water has different densities. High-density water at around 4 degrees Celsius separates from the low-density water at high temperatures. However, when the season changes and the water becomes uniformly the same temperature, all the layers of the water can mix, which is called a ‘turn over.’

What do these results mean?
In science, we need to test to confirm our hypothesis. Kat, our founder, borrowed a device that would take a sample from the bottom of the lake from some lake monitoring experts. The water she collected was coloured red and smelled of sulfur. Lab results showed high levels of compounds that confirmed the lake wasn’t turning over. She then asked experts to review the data. Three experts confirmed that the profiles are typical of a meromictic lake.
Since lake McGlashan does not fully turn over, it’s rare: it’s why we call it a meromictic lake! It also makes it more sensitive to human impacts, especially excess waves from motor boats.
We always say, “every body of water has its own unique chemistry,” and in the case of Lac McGlashan, it’s truly one in a thousand!
Dive deeper
This article was written by Peter McClaren (Biology Student, University of Ottawa) with edits and updates by Kat Kavanagh (Executive Director of Water Rangers, Masters of Science from McGill University).